(Click here for past publications):
Nanomechanics of repeat proteins
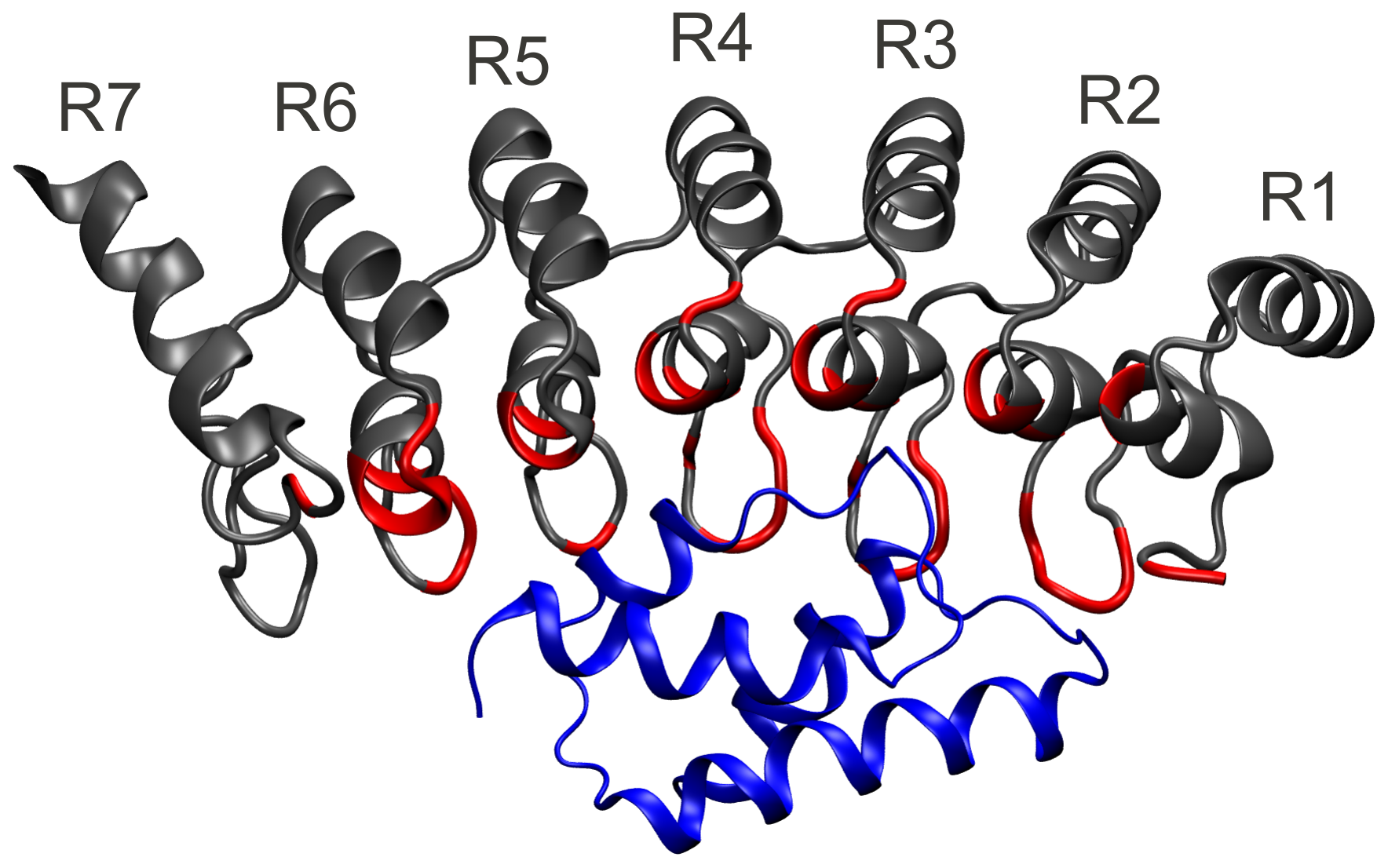
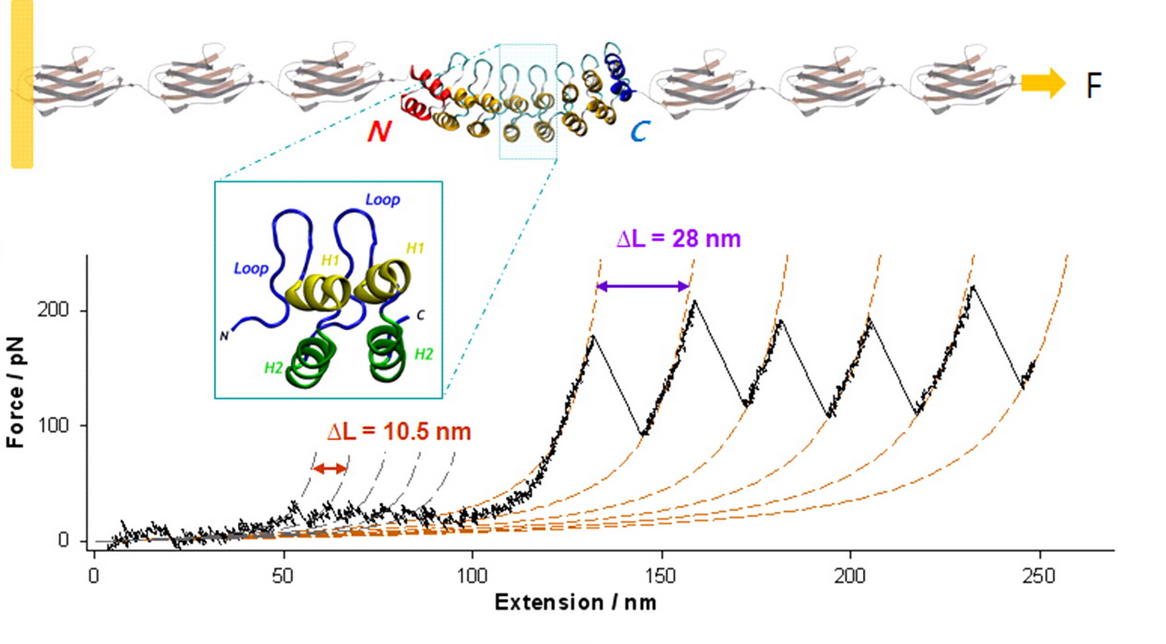
We primarily use AFM-based single molecule force spectroscopy (SMFS) and molecular dynamics simulations to examine the mechanical and folding behavior of wild type ankyrin repeat proteins and their mutants.
Selected Publications:
- Capturing the mechanical unfolding pathway of a large protein with coiled-coil probes Angew. Chem. Int. Ed., 2014.
- Effect of ligand binding on Ankyrin repeats PLOS Comp. Bio., 2013.
- Anisotropy of ankyrin repeats Biophys. J., 2012.
- Forceful refolding of repeat proteins Biophys. J., 2010.
- Nanospring behavior of ankyrin repeat proteins Nature, 2006.
Folding of proteins and nascent chains
We use AFM imaging, AFM pulling, in vivo and in vitro protein expression to examine the folding of proteins at the single-molecule level.
Selected Publications:
- Chaperones Rescue Luciferase Folding by Separating its Domains JBC, 2014.
- AFM imaging of ribosome nascent chains Chemm. Com., 2012.
- Reconstruction of vectorial folding pathway of Ankyrin repeats J. Bio. Chem., 2010.
DNA mechanics
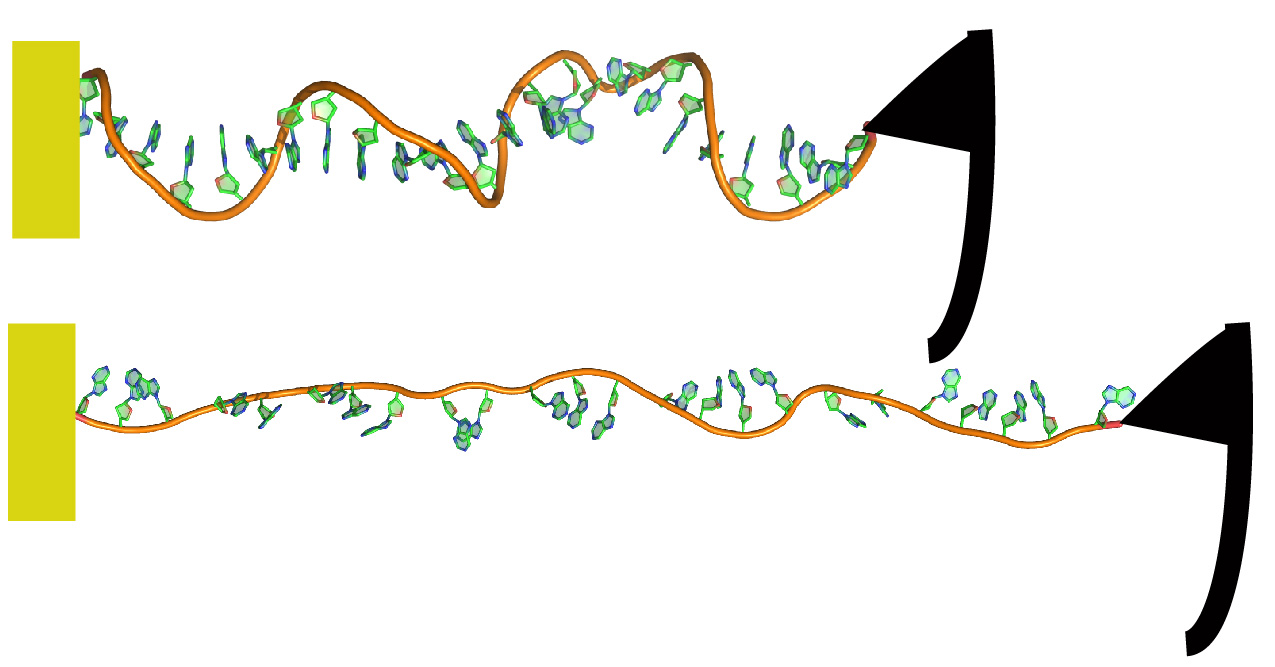
We use AFM and computational approaches to investigate the elasticity of single-stranded DNA and force-induced conformational transitions in ssDNA.
Selected Publications:
- Origin of overstretching transition in single-stranded DNA PRL, 2013.
- Direct measurements of base-stacking PRL, 2007.
DNA mismatch & repair

We adapt and develop various single-molecule approaches to study the interaction of MMR proteins among themselves and with heteroduplex DNA to elucidate the mechanism of MMR initiation.
Selected Publications:
- MutS tetramerization captured by AFM The EMBO J., 2011.
- Detection of UV damage in single DNA molecules Biophys. J., 2007
AFM improvement
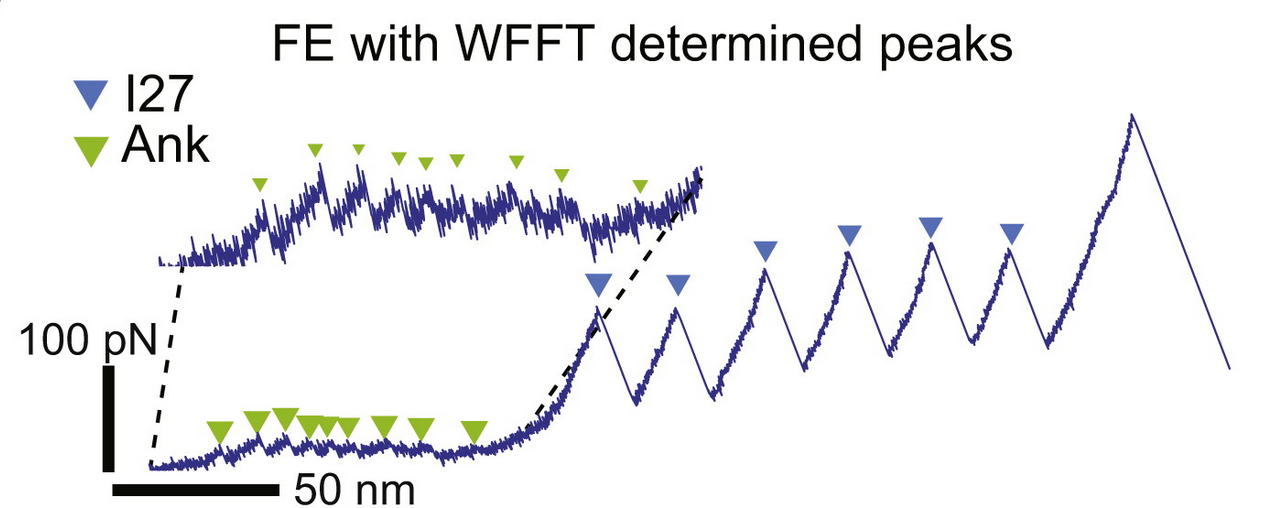
We have been working to make it easier for unbiased selection of data and have developed some software which can be accessed here.
- Automated real-time data collection Ultramicroscopy, 2014
- Calibration of lateral force measurements Nanotech., 2013
- Pulling geometry-induced errors in SMFS Biophys. J., 2007