Context
Olfaction, the sense of smell, is one of the five fundamental senses that enable human beings to perceive and make sense of their surroundings. Amid the COVID-19 pandemic, the loss of the sense of smell had profound repercussions on daily life. This sense not only plays a pivotal role in helping individuals make food choices, shaping taste experiences, and impacting social interactions but also serves as an essential early warning system for potential hazards like fires and gas leaks. For years, scientists have dedicated their efforts to gaining a deeper understanding of the intricacies of olfaction.
The existing literature presents two conflicting models to explain the mechanism of olfaction. The first model, referred to as the "Shape Theory of Olfaction (STO)," suggests that odorants are perceived by olfactory receptors (ORs) through a mechanism similar to a key fitting into a lock. The second model, rooted in quantum mechanics and known as the "Vibrational Theory of Olfaction (VTO)," associates the scent of molecules with their vibrational characteristics and proposes that odor recognition involves a quantum mechanical process known as inelastic electron tunneling spectroscopy where electrons tunnel inelastically through the odorant-bound receptor. However, it's worth noting that both theories lack conclusive evidence and remain topics of controversy and ongoing research.
Experimental setup
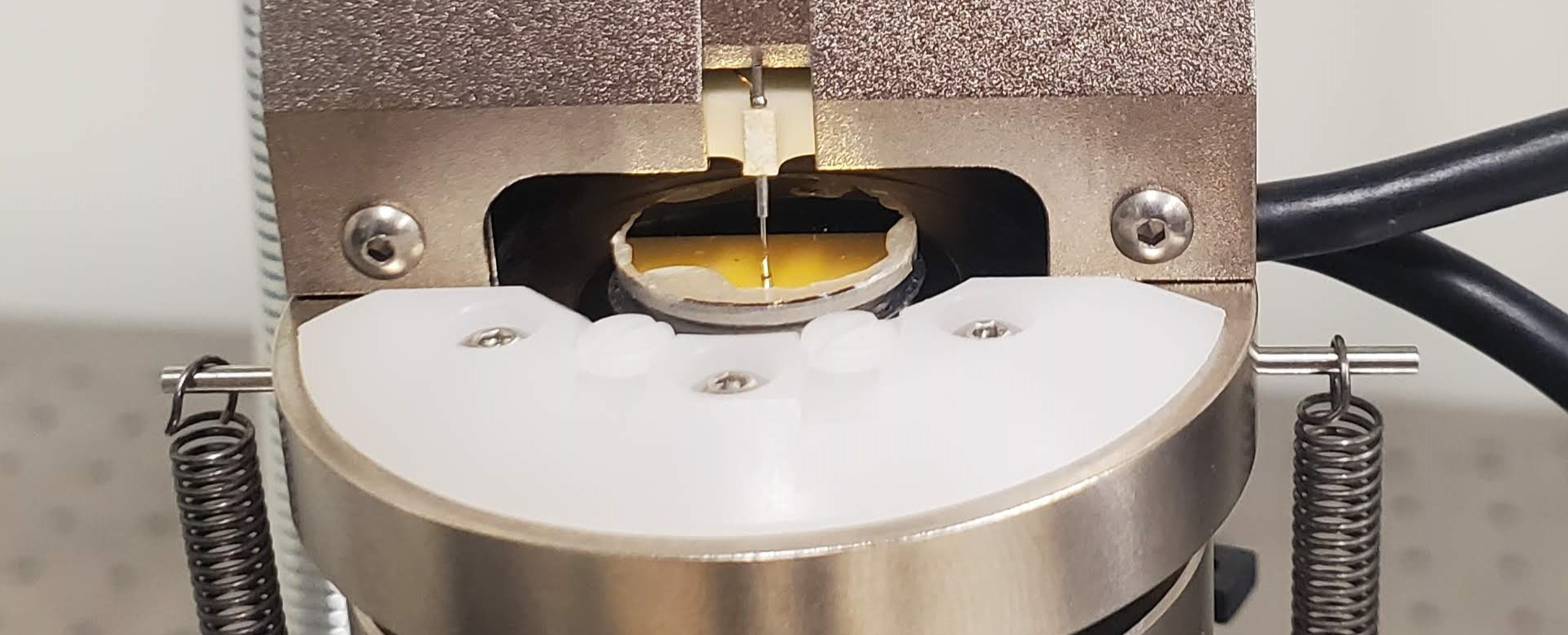
In this project, our primary aim is to perform tunneling measurements using a variety of odorants. Our laboratory is equipped with a versatile multi-mode atomic force microscope (AFM). This instrument enables us to not only characterize our surfaces using AFM mode but also to measure tunneling current through the employment of the Scanning Tunneling Microscope (STM) mode.
We have mounted the system on an optical table to effectively isolate the experiment from environmental vibrations. After conducting numerous tests, we have determined that it is necessary to enhance the vibration-damping capabilities further. As a result, we constructed an additional isolation system to achieve this goal.
The STM head is affixed to both an MDF plate and an aluminum plate, which are suspended by three 2-foot-long springs. This arrangement results in the creation of a pendulum with a 1 Hz resonant frequency, effectively isolating the system from horizontal vibrations, and a harmonic oscillator with a 2 Hz resonant frequency, providing protection against vertical vibrations.
Furthermore, we have introduced three sets of three hard drive magnets to the base. When brought into close proximity to the aluminum plate, these magnets induce eddy-current damping. As the aluminum plate moves toward the magnets in any direction, the changing magnetic field experienced by the plate triggers the generation of eddy currents within it. These eddy currents, in turn, produce a magnetic field that opposes the plate's movement, either by attracting or repelling the hard drive magnets, thereby efficiently reducing vibrations.
For more details please visit Dr. Dan Berard website:
Scanning tunneling microscopy STM
The scanning tunneling microscope (STM) operates based on the concept of establishing a tunnel junction between a metal tip and a sample, with an insulating barrier, the vacuum, separating them. The thickness of this barrier, denoted as d, is typically on the scale of Angstroms (Å). The transfer of charge between electrodes occurs through tunneling when the distance between the tip and the sample is reduced to a significant extent. In this scenario, the electron behaves as an evanescent wave at the barrier, exhibiting exponential decay over the distance d.
One of the basic functions of the scanning tunneling microscope is to map the surface of a sample. It is essential to avoid contamination and degradation of the atomic layers on the surface of the material. We present here some results obtained for different samples.
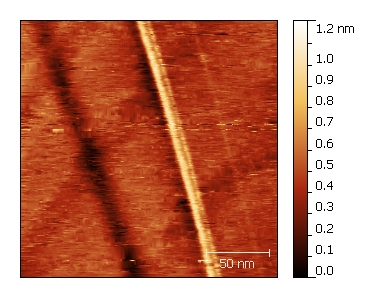
Graphite
In order to have a clean surface for scanning tunneling microscopy, we use the scotch tape method. This technique known as mechanical exfoliation involves using adhesive tape to repeatedly peel layers of graphite. A clean surface is then ready to be used. The sample is positioned on a magnetic disc, and electrical contact is ensured by applying silver paste.
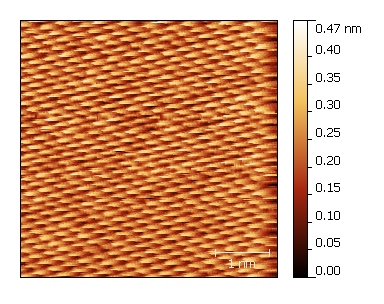
Gold
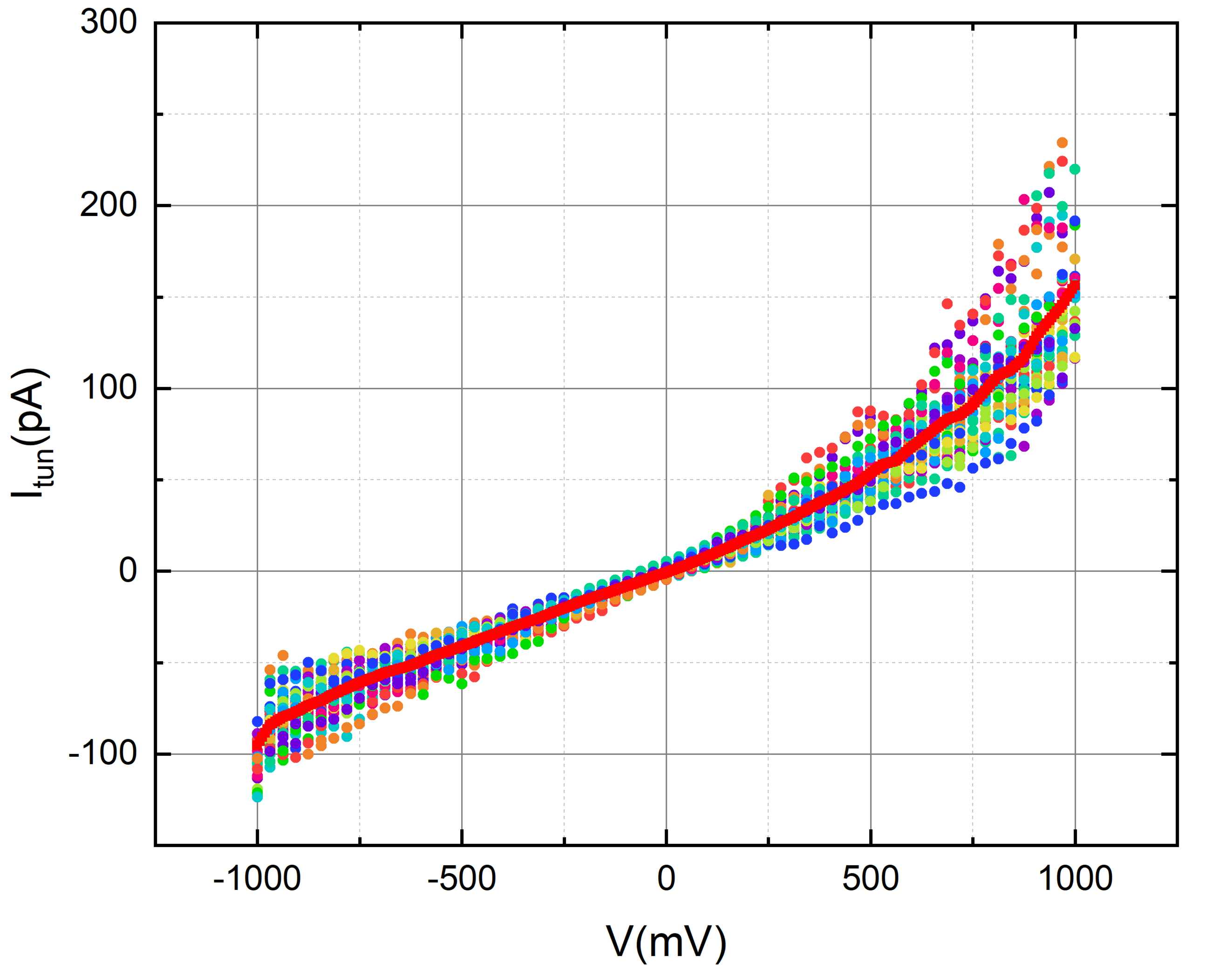
We use glass discs coated with evaporated thin films of gold. To enhance the adhesion of the 250 nm thick gold layers, we incorporate an intermediary chromium layer measuring 20 nm.
Octanal (C8H16O)
Octanal, also known as octanaldehyde or caprylaldehyde, is an organic compound with the chemical formula C8H16O. It belongs to the class of aldehydes, which are organic compounds containing a carbonyl group (C=O) with a hydrogen atom and an R group attached. In the case of octanal, the R group is an eight-carbon linear alkyl chain. Octanal contributes to the characteristic odor of these oils and is used in the fragrance and flavor industries. The compound has a strong, citrus-like odor and is often described as having a fruity and orange-like scent.
It's worth noting that octanal, along with other aldehydes, is involved in the field of olfaction (the sense of smell), and its scent is an area of interest in studies related to aromas and fragrances.
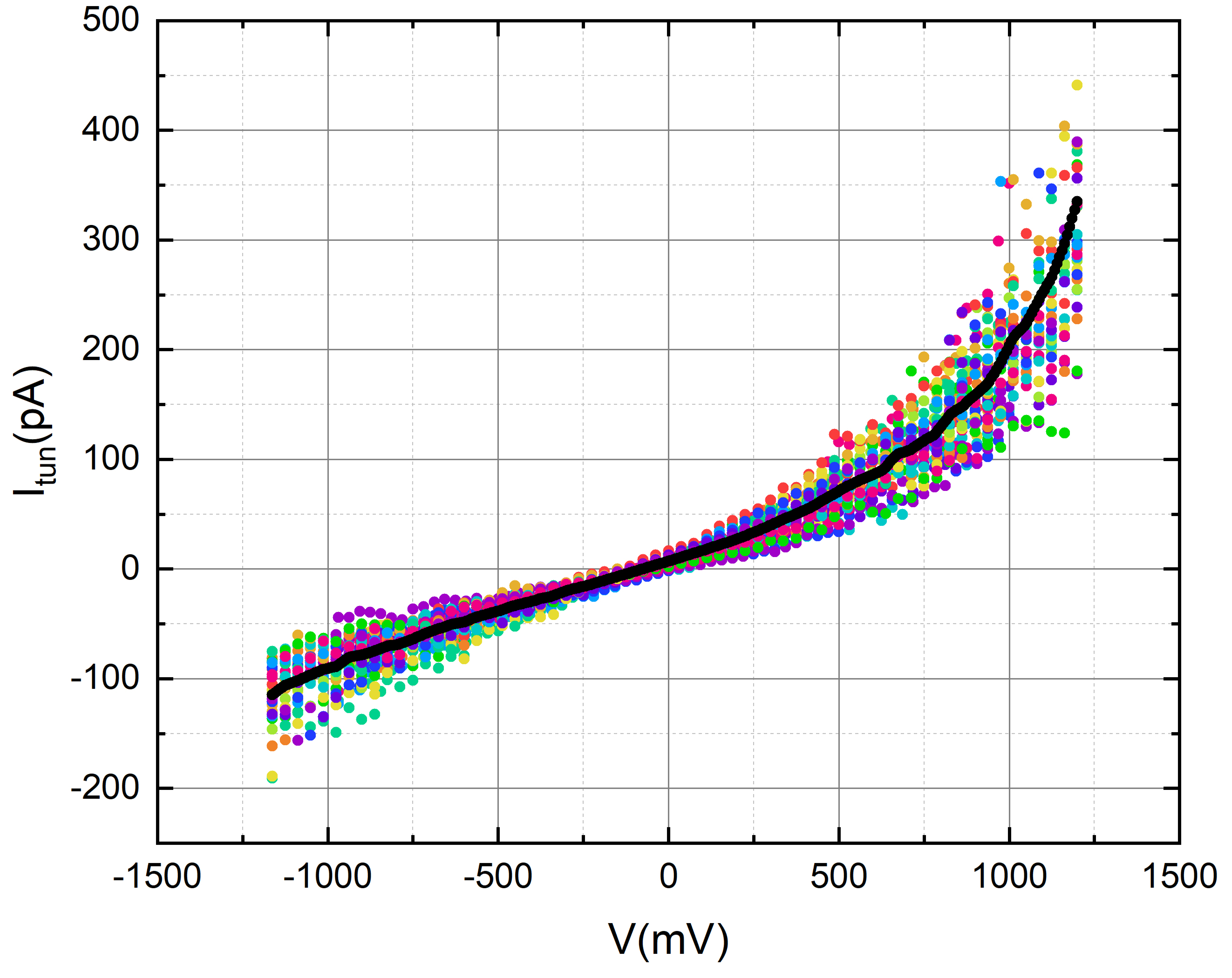
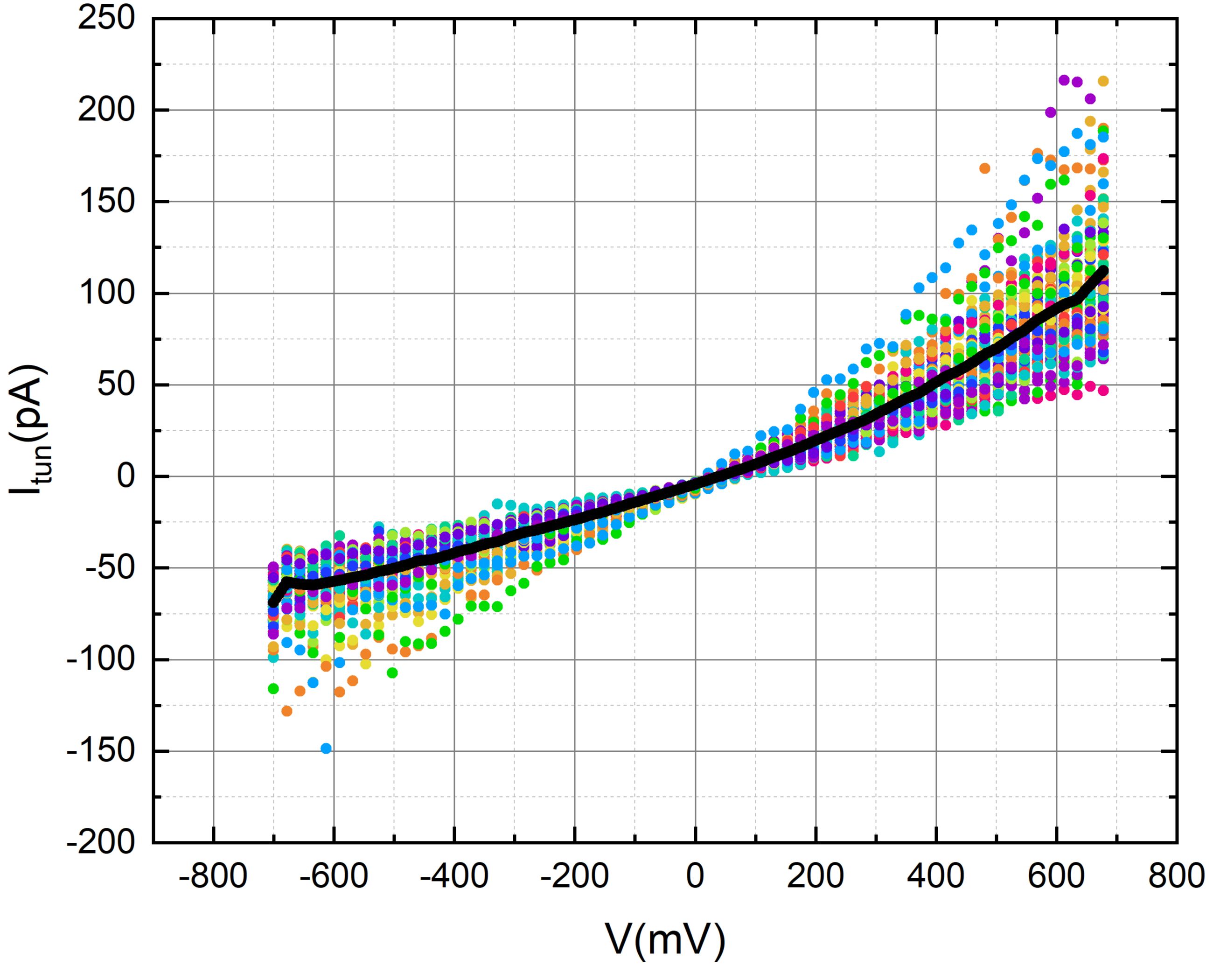
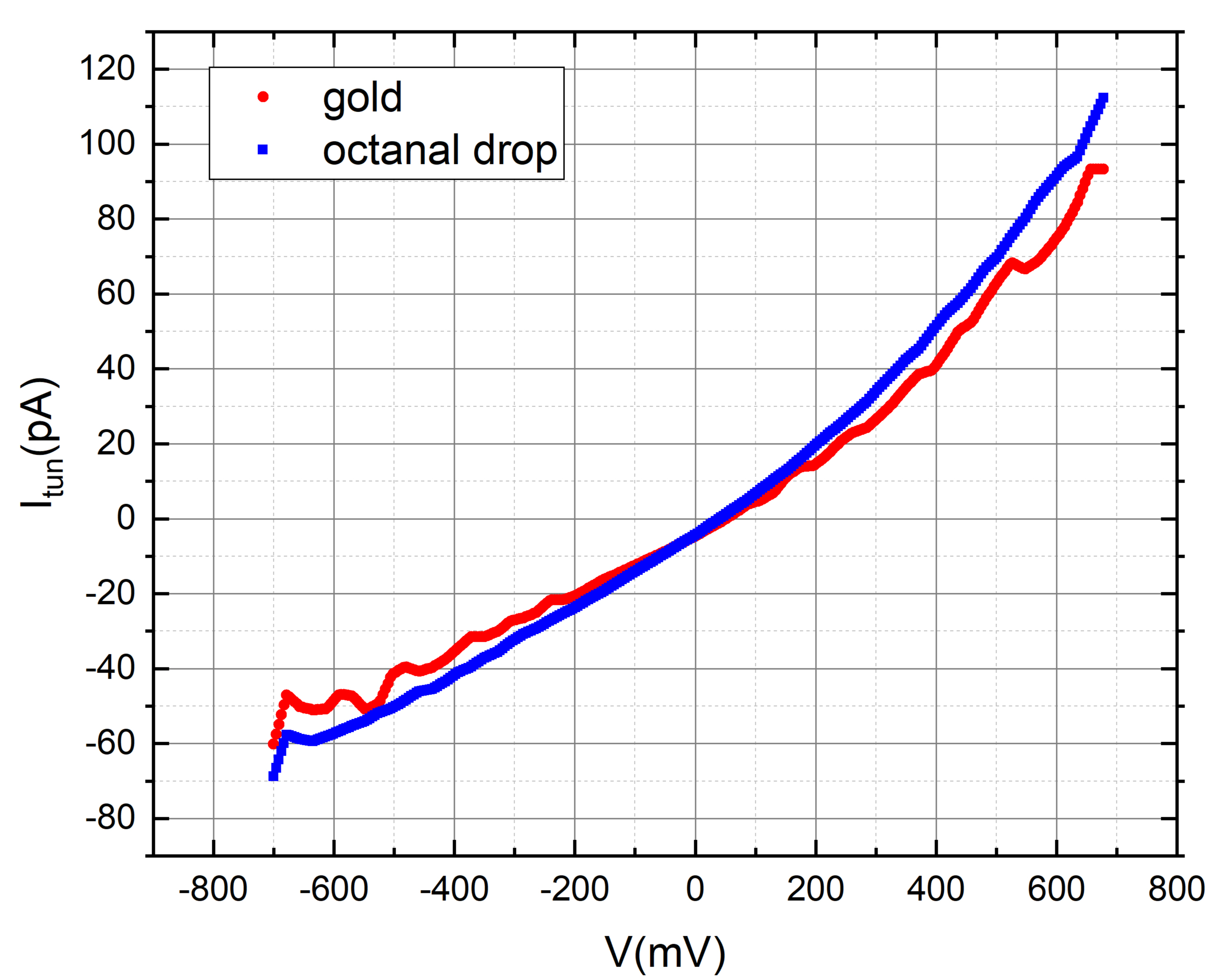
We employed octanal to assess the tunneling current of gold in the presence of its aroma. To accomplish this, we meticulously applied a 2-micro liter droplet of octanal onto the gold disc, ensuring careful handling to avoid contact with the tip.
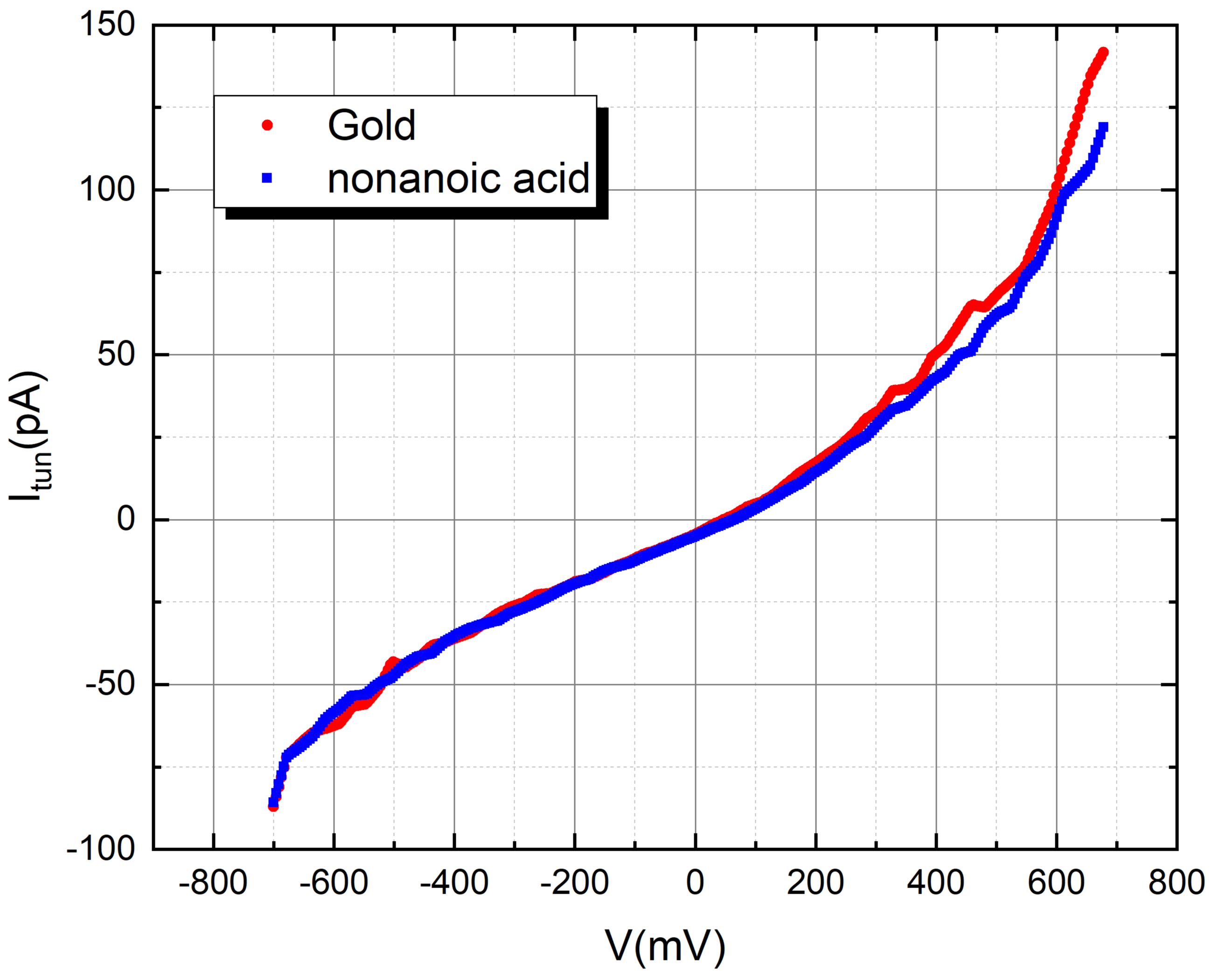
Nonanoic acid (C9H18O2)
Nonanoic acid, also known as pelargonic acid, is a nine-carbon linear chain fatty acid.It is a saturated fatty acid, meaning that it contains only single bonds between carbon atoms. The name "nonanoic" is derived from "nona-" meaning nine and "-anoic" indicating the presence of a carboxylic acid functional group.
Nonanoic acid is found naturally in some foods, particularly in trace amounts in certain plants and essential oils. It is used in various applications, including the production of esters for flavor and fragrance purposes. The compound has a pungent and unpleasant odor.
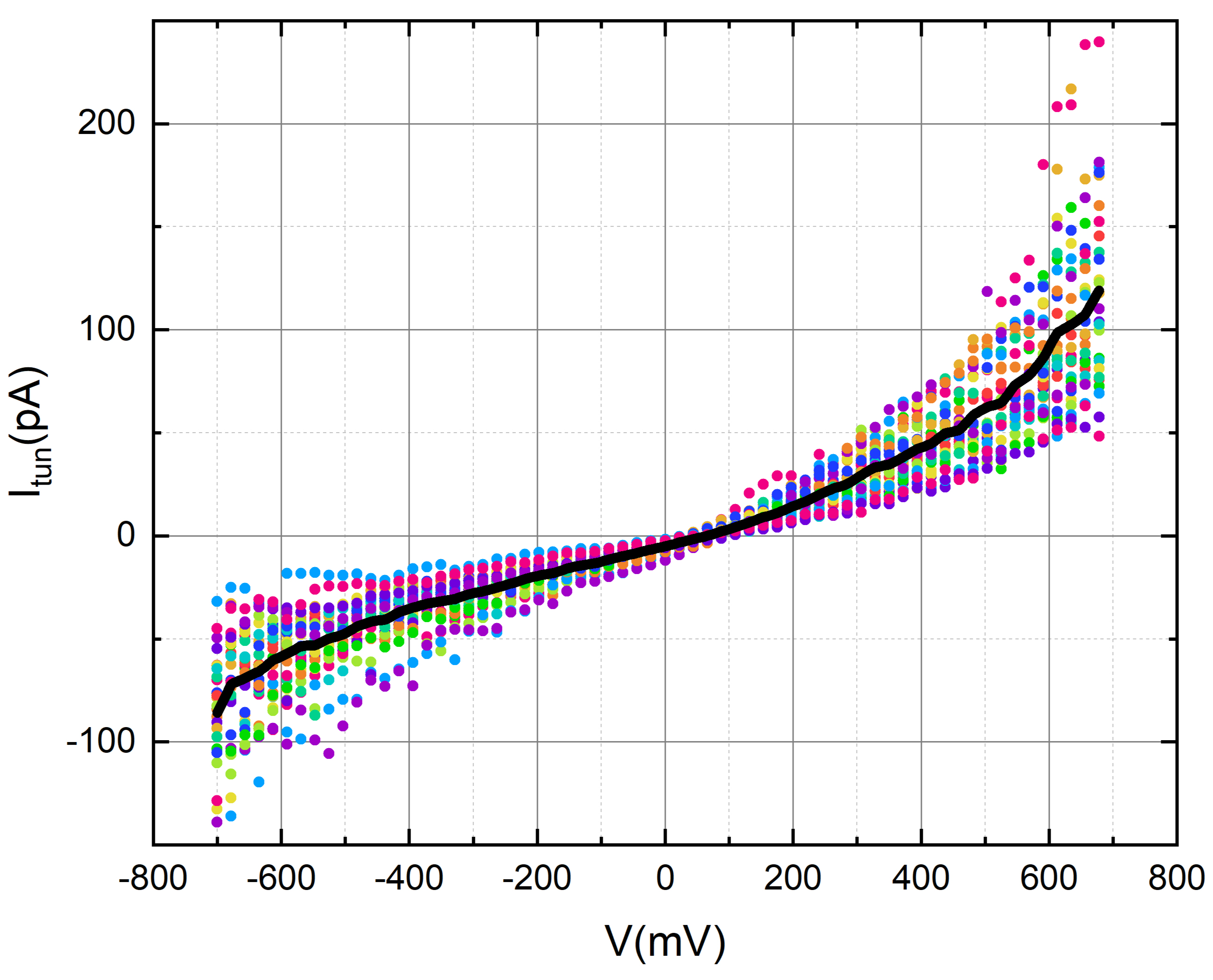
We used nonanoic acid to evaluate the tunneling current of gold while exposed to its fragrance. To achieve this, we carefully administered a 2-micro liter droplet of nonanoic acid onto the gold disc, taking precautionary measures to avoid any contact with the tip.
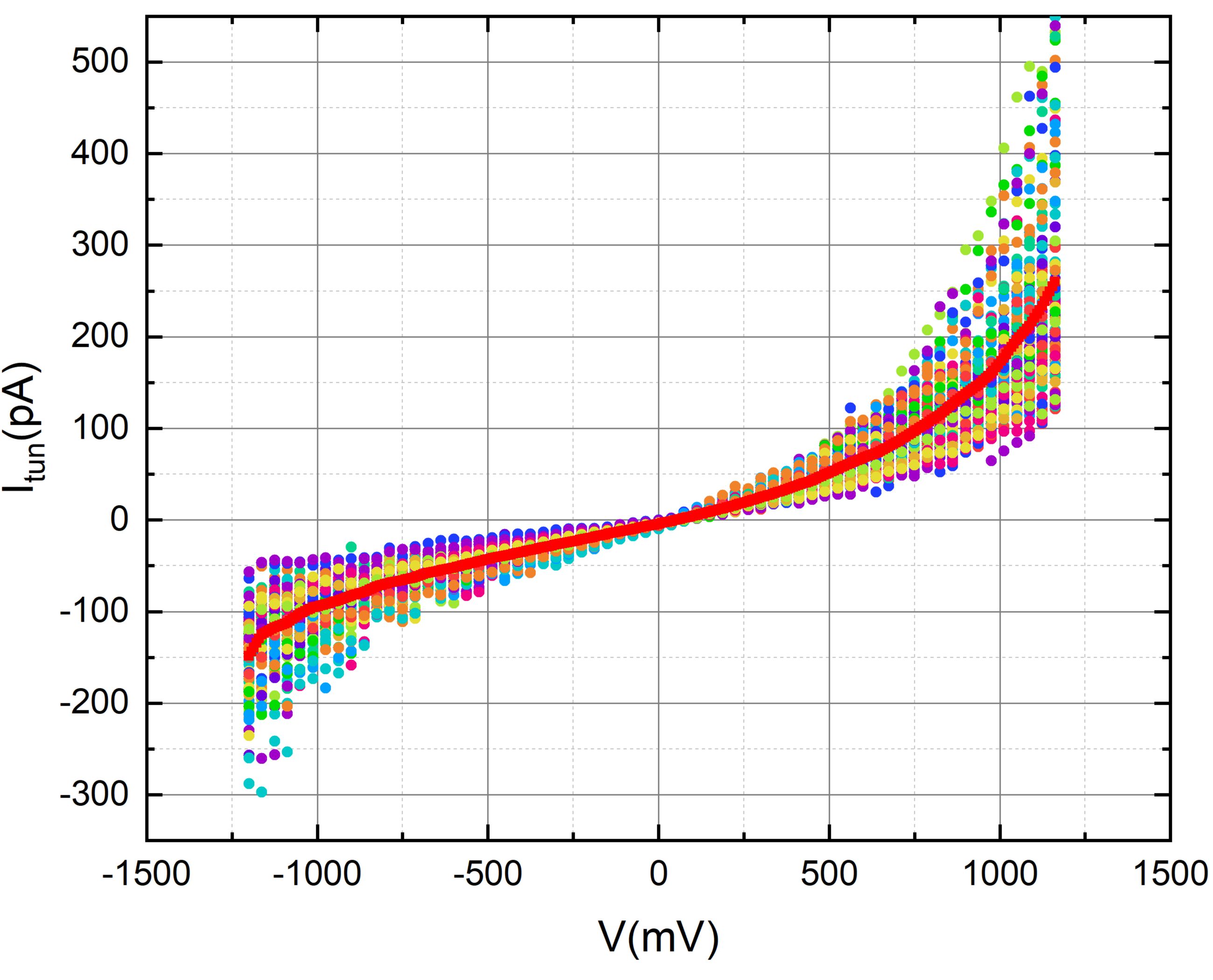
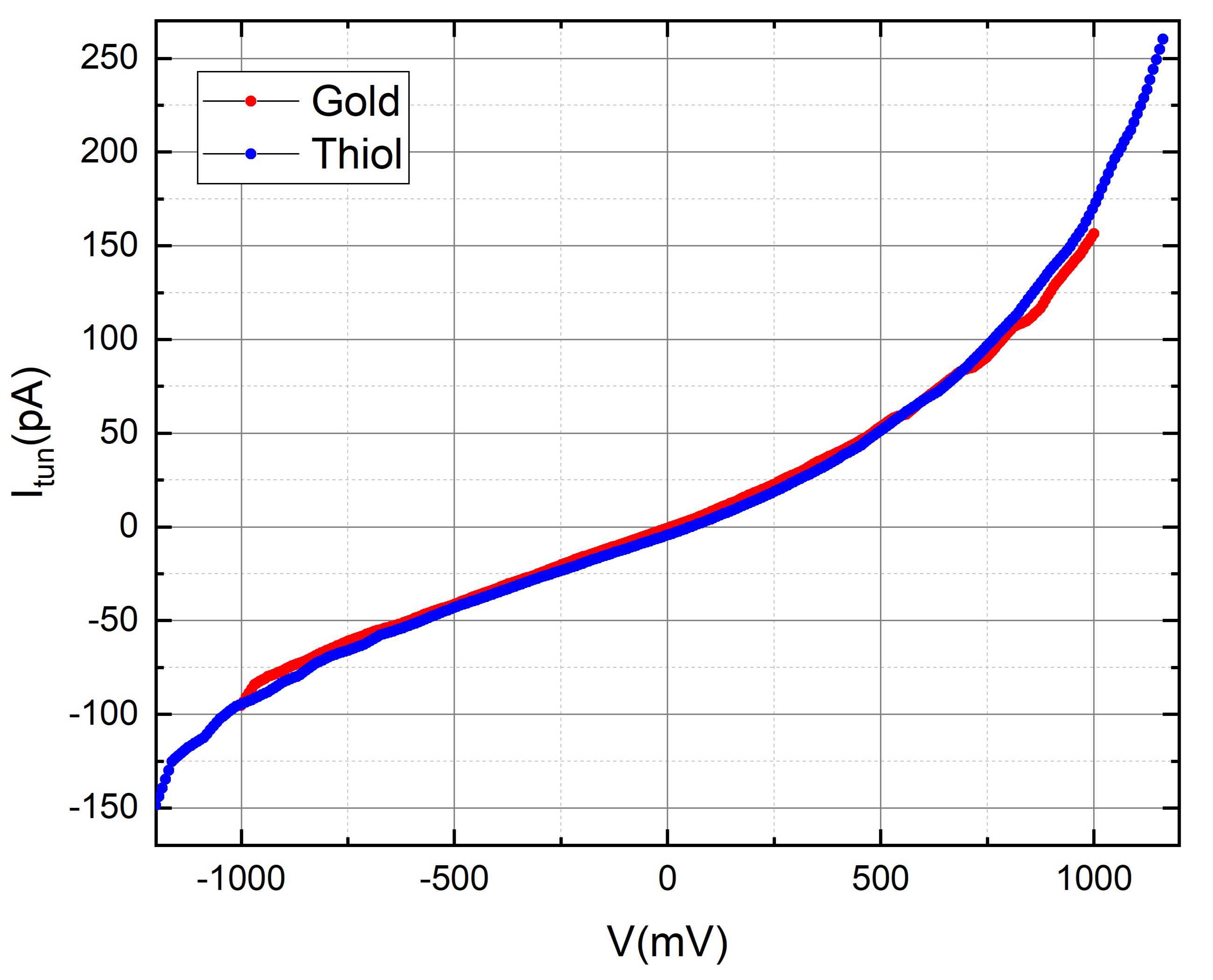
Thiol
A thiol is a type of organic compound that contains a sulfhydryl (–SH) functional group, which consists of a sulfur atom bonded to a hydrogen atom.
In the context of olfaction, thiols play a significant role in contributing to the characteristic odors of various substances. Thiols, with their distinctive sulfur-containing functional group (–SH), are known for producing strong and often unpleasant smells. The human nose is particularly sensitive to the presence of thiols in certain compounds.
Self-assembled monolayers (SAMs) of thiols on gold surfaces are a well-established and extensively studied system in the field of surface science and nanotechnology. This involves the spontaneous organization of thiol molecules on a gold surface to form a single layer.
We prepared self-assembled monolayers (SAMs) using 11-alkanethiols on thin gold films. We modified the procedure outlined in this article by introducing 3.5 μL of undecanethiol into 5 mL of ethanol. Subsequently, the solution underwent sonication for 5 minutes. Placing the glass disc with the gold side facing up in the vial, we degassed the solution overnight by bubbling N2, facilitating SAM formation. The solvent on the SAM was then carefully evaporated by gently blowing a stream of nitrogen over the chip. Measurements on the chip were conducted within 2.5 hours of SAM formation.
Discussion
Our measurements consistently depict a tunneling current profile that, at the current resolution and level of measurement noise, shows no noticeable variations linked to the presence of the odorant. Nevertheless, these results provide a positive groundwork, laying the groundwork for future studies on how low pressure (vacuum) and low-temperature impact the sensitivity of the tunneling current to odorant molecules when the noise level is substantially decreased.
Acknowledgment: This work was supported by a National Science Foundation (USA) grant MCB 2105612.